
A Clear and Simple Definition of Quantum in the Year of Quantum
According to the foundational definition quantum signifies "small and discrete packets,".
This definition of energy in the universe aligns with the classical idea that everything is composed of smaller components. For instance, molecules are composed of atoms, like the atoms are made up of protons, neutrons, and electrons. Similarly, photons are discrete packets that form part of larger entities such as electrons, protons, and neutrons.
The photon can be defined as the smallest, lightest, and fastest packet that traverses the universe, existing in all galaxies and circulating throughout the cosmos. The standard quantum energy is essentially the photon, whose energy is calculated using Planck's relation (E=hϑ).
It is important to note that the energy of a quantum packet is highly variable and depends on its frequency. For example, considering a frequency range from 300 THz to 900 THz, the energy quanta in this narrow band of the electromagnetic spectrum can vary significantly.
To define a standard unit, it must be constant. In the SI system, the unit of length is one meter, which is a fixed measure, and the unit of mass is one kilogram, also a fixed measure. Therefore, to define a standard quantum of energy, we must introduce a unit with a constant value.
Previous articles have demonstrated that the mass and speed of a photon are constant. If we aim to define the fundamental unit of quantum energy, it can be expressed as follow:
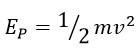
This general definition applies to any amount of kinetic energy, and according to previous calculations, the true and actual speed of a photon is “3.3 C”. Consequently, the energy of a photon can be calculated as follows:
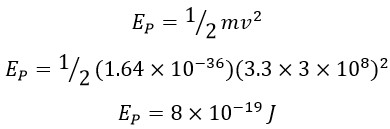
This constant value can be referred to as the standard quantum energy in the universe:
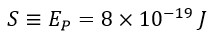
This introduced standard value for all electromagnetic waves is a fixed, enduring, and universal quantum with specific characteristics, meaning that:
1. This energy value is constant.
2. It is governed by mathematical and physical equations.
3. The nature of this energy is well-defined and represents the energy of a single photon.
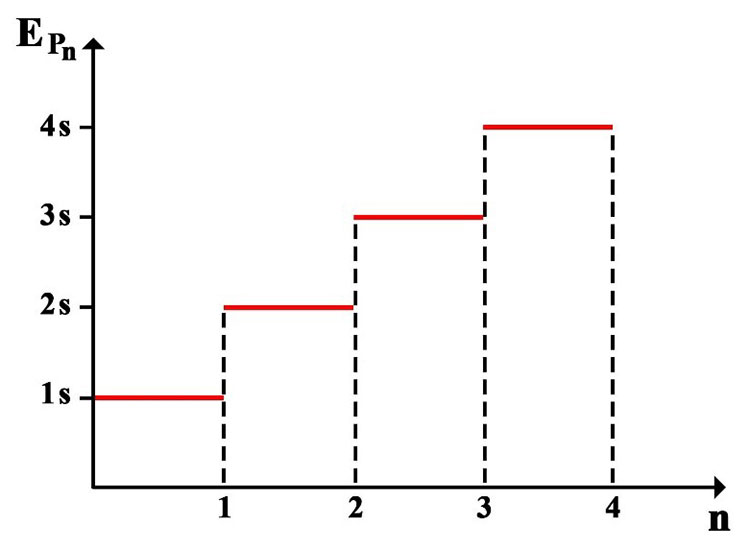
4. For larger quantities of energy, the term “nS” can be used, where “S” is the energy unit of a single photon and “n” is the number of independent photons in the set.
Based on the above explanations, quantum energy diagrams can be represented as follows:
Notice:
In general, the energy of a single quantum packet (a single photon) and a set of n quantum packets could be represented as:
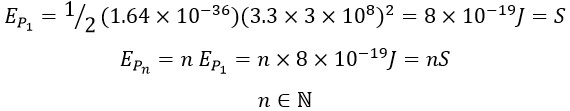
 Download PDF
Download PDF 

