A New Explanation for the Formation and Structure of Orbitals (1s2) in the Universe 2024, Part B
1. Calculation of Frequencies, Wavelengths, Amplitudes, and Properties of Electrons and Protons
2. New Explanation for Color Variety of Electrons in Various Trajectories
3. The Destructive Impact of Electrons in Various Trajectories
1. Calculation of Frequencies, Wavelengths, Amplitudes, and Properties of Electrons and Protons
In light of the previous articles, the electron's movement around the nucleus is a combination of several types of motions:
I. Planet-Like Motion: The electron orbits around the nucleus like a planet. This is a simple motion, and Newtonian laws govern it.
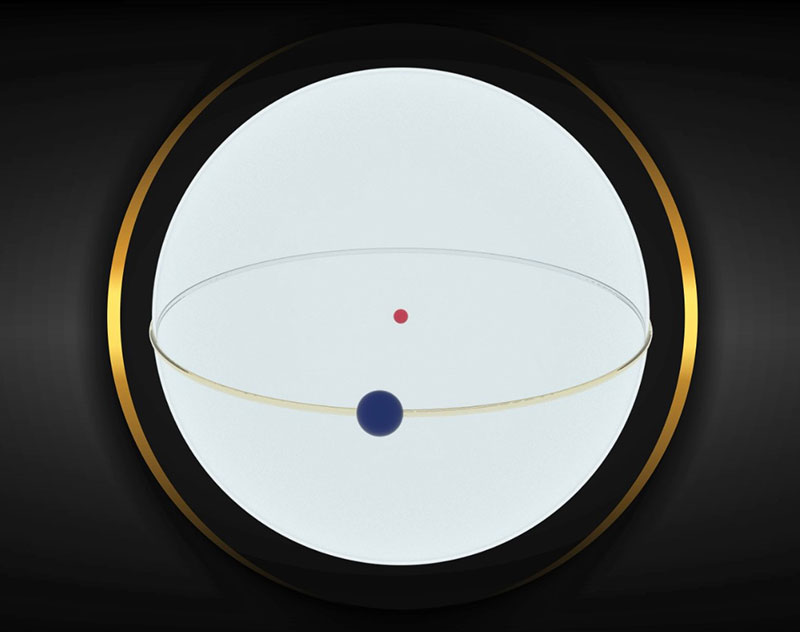
II. Closed Helical Path Motion: As previously stated, "each electron revolves around itself at a speed close to the speed of light." This motion causes curvature in the path of the solar motion of the electron, transforming its path around the nucleus into a closed helix. Note that this motion is a back-and-forth movement.

III. Spherical Motion Around the Nucleus: Atomic nuclei carry a positive charge, while electrons carry a negative charge. It can be said that atomic nuclei affect electrons. On the other hand, atomic nuclei rotate around themselves at a speed close to the speed of light. This rotation causes an additional rotational motion to be added to the helical path of electron motion. Consequently, the electron is rotating around the nucleus and sweeping the entire surface of a sphere with an atomic radius.
a. Calculating the Frequency of Electron of Hydrogen Atom
Given the helical path of electron motion around the nucleus, the frequency of its motion can be determined using the following equations and a straightforward method:
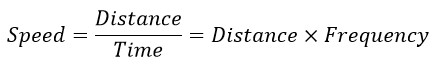
So, in general, it can be said:
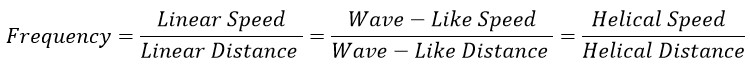
Therefore, the frequency of a single electron in a hydrogen atom can be calculated as follows:
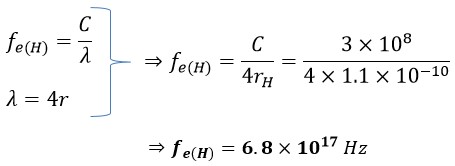
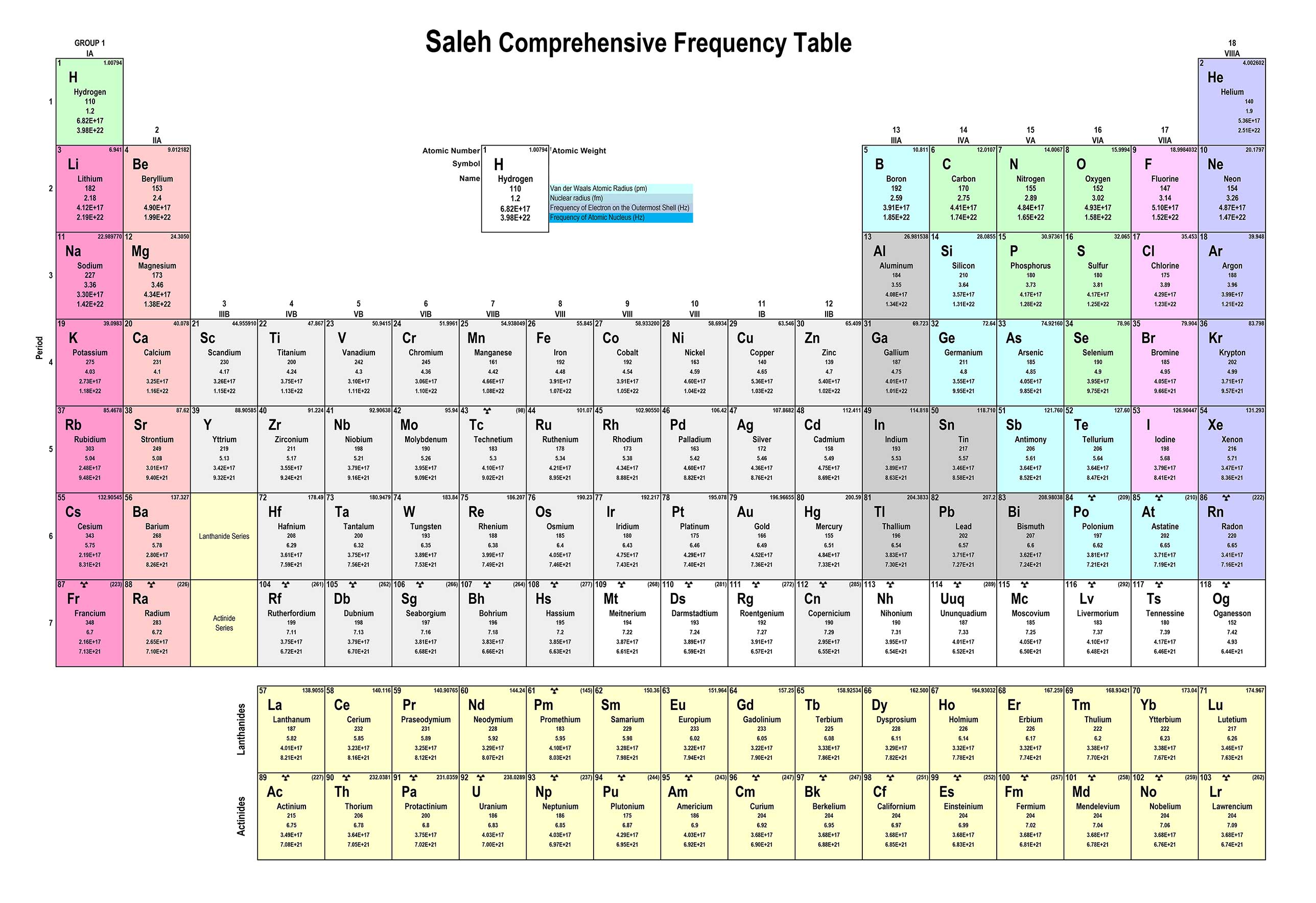
In this context, 𝛌 represents the wavelength, C is the speed of light, and fe(H) denotes the frequency of the electron in the hydrogen atom. The parameter rH, equivalent to the radius, corresponds to the atomic radius of hydrogen. As another illustrative example, let's calculate the electron frequency for the outermost layer of a Gold atom:
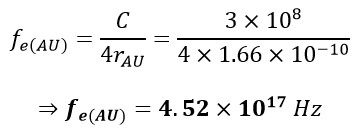
b. Calculating of the Frequency of Proton of Hydrogen Atom
It is quite clear that the Moon orbits around itself and around the Earth, which is itself rotating around its axis and around the Sun. The Sun, in turn, orbits around itself and around the central black hole of the Milky Way galaxy. The black hole is also in a state of rotation. Additionally, electrons orbit around themselves and around the nuclei of atoms. The nuclei, too, rotate around themselves at the speed of light [1]. Therefore, one can write a repeatable sinusoidal equation for the rotation of a proton around itself:
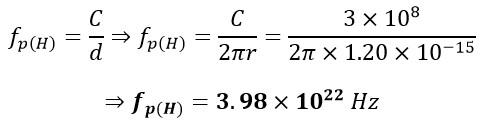
In which r is the radius, and fp(H) is the frequency of proton of the hydrogen atom. Now, let's proceed to calculate the frequency of the atomic nucleus of Gold fp(AU):
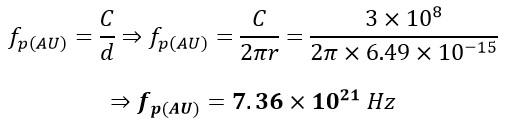
2. New Explanation for Color Variety of Electrons in Various Trajectories
Sometimes, electrons can be observed freely in nature; such as a spark generated from a wire, a lightning strike in nature, etc. However, upon closer inspection, they appear in various colors like yellow, blue, white, and so on. The reason for this color variety lies in the different frequencies of electrons in their various states. For instance, the frequency of lightning which produces colors like white, whereas the frequency of electrons emitted from a wire, typically displaying hues such as red and blue, is different. Essentially, different electron frequencies generate different colors.

3. The Destructive Impact of Electrons in Various Trajectories
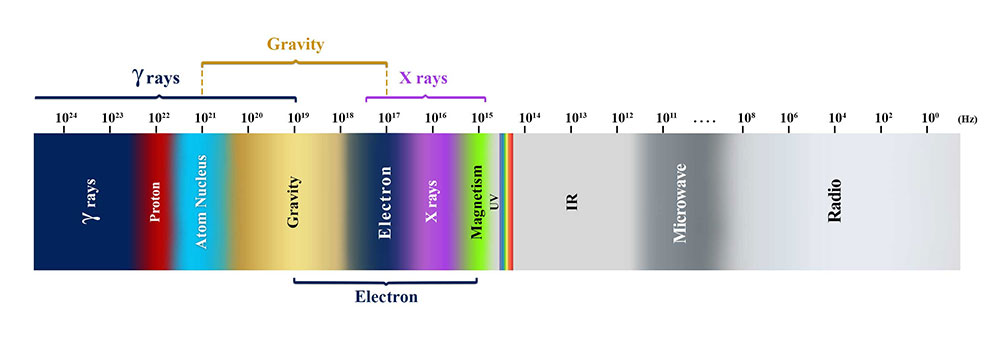
Given that the mass and speed of electron, among other factors, remain constant, its manifestation as lightning leads to severe destruction. In household electrical currents, this destruction is moderate, and in photoelectric currents, it is weak. The various effects of these types of currents can be attributed to the number of electrons present as well as their different frequencies. For instance, the approximate range of frequencies of electrons in photoelectric currents is around 1015 Hz, in household electrical currents is about 1016 Hz, for free electrons, it is 1017 Hz, in lightning is 1018 Hz, and for magnetars is about 1019 Hz.